Introduction
Autism Spectrum Disorder (ASD) is a behavioral/developmental disorder characterized clinically by delays and qualitative differences in communication and social interactions well as repetitive behaviors and restricted interests. Currently, it is a subjective psychiatric diagnosis based on behaviors exhibited in the child rather than an objective medical diagnosis based on core clinical imbalances resulting in abnormal behaviors. This subtle but powerful difference in diagnosing patients with autism has resulted in profound effects on the results of medical trials. Many trials have been plagued with inconsistent results because patients are being selected based on the behaviors they exhibit rather than known clinical imbalances they possess which cause or contribute to the behaviors.
There is no single cause of autism. Instead, the causes are as varied and diverse as the individuals who are affected. This is the primary reason why the pharmaceutical industry has failed to produce any effective treatments beyond simple symptom control (such as antipsychotics for agitation or stimulants for inattention).
 Your child’s conventional medical doctor has probably done very little for your child because they have been trained that there is no cure for autism and your child’s symptoms and behaviors are a result of their autism. The diagnosis of autism in your child was made subjectively, based on the symptoms and behaviors they exhibited. This reasoning dictates that the symptoms define the disease (behaviors therefore autism) and the disease causes the symptoms (autism therefore behaviors). This is a logical fallacy called circular cause and consequence. With this illogical thinking, its no wonder conventional medicine has almost nothing to offer our children with autism. Autism is not an entity which bites your child and then causes disease – it is just a convenient name to place individuals exhibiting similar behaviors stemming from a multitude of different physiological insults and imbalances. Language may guide thought, but names do not cause disease.
Your child’s conventional medical doctor has probably done very little for your child because they have been trained that there is no cure for autism and your child’s symptoms and behaviors are a result of their autism. The diagnosis of autism in your child was made subjectively, based on the symptoms and behaviors they exhibited. This reasoning dictates that the symptoms define the disease (behaviors therefore autism) and the disease causes the symptoms (autism therefore behaviors). This is a logical fallacy called circular cause and consequence. With this illogical thinking, its no wonder conventional medicine has almost nothing to offer our children with autism. Autism is not an entity which bites your child and then causes disease – it is just a convenient name to place individuals exhibiting similar behaviors stemming from a multitude of different physiological insults and imbalances. Language may guide thought, but names do not cause disease.
Attention Deficit Hyperactivity Disorder (ADHD) and Autism Spectrum Disorder (ASD)
From a functional medicine perspective, there is much overlap between these two disorders. Both are neurodevelopmental disorders, both can be functionally debilitating for the affected individual and both can be greatly improved, or even overcome, with the correct balancing of systems and appropriate neurological stimulation (various therapies).
Many of the biomedical approaches which are successful in ASD are also beneficial for ADHD. This is no coincidence because the root cause of both disorders is neurological dysfunction secondary to many of the clinical imbalances described below. The way the individual expresses this neurological dysfunction and how it impacts their daily functioning is usually a matter of severity. Typically, the level of neurological deficit is significantly less in individuals with ADHD compared ASD, typically resulting in a more rapid and dramatic response to treatment.
The Role of Genetics
 About 6-10% of patient with ASD have Syndromic Autism. Syndromic Autism is defined as autism secondary to an existing genetic syndrome such as Fragile X Syndrome, Down Syndrome or Duchenne’s muscular dystrophy, to name a few. For a more complete list of syndromes click here.
About 6-10% of patient with ASD have Syndromic Autism. Syndromic Autism is defined as autism secondary to an existing genetic syndrome such as Fragile X Syndrome, Down Syndrome or Duchenne’s muscular dystrophy, to name a few. For a more complete list of syndromes click here.
Many of the biochemical contributing factors may still be present in these patients, however the response to treatment may not be as successful as in Non-syndromic Autism.
Non-syndromic Autism comprises the vast majority of ASD individuals (90-94%). They have no definitive genetic cause for their autism. However, many of them will have genetic mutations which play a significant role in the contributing factors listed below (i.e. MTHFR, FOLR1, DHFR, GSTM1 etc.)
Over the past decade, conventional medicine has directed much of its research efforts and funding towards identifying the gene or genes responsible for autism. Billions of dollars have been spent with no substantial changes in the proportion of patients identified with a clear-cut genetic cause. This misdirection of research resources has resulted in a grave disservice to our children with autism. Future generations of children with autism may possibly benefit from this research but the current generation is being marginalized by resource allocation decisions of our current conventional medical model. Treatments are available for our kids today. With the availability of adequate research dollars, these treatments could be refined and improved at a much quicker pace than is happening today.
Our Approach
The Autism Biomed Center program is based on the foundational principles of Functional Medicine and protocols developed by the faculty of the Medical Academy of Pediatric Special Needs

Our approach asks two keys questions:
1. Is the patient’s body and brain getting what they need to function optimally (i.e. vitamins, minerals, omega-3 fatty acids, healthy clean food, etc.)?
Ensure the good stuff gets in.
2. Is something present in the patient’s body and brain that is interfering with their ability to function optimally (i.e. toxins, low grade infection, disrupted microbiome, free radicals, cytokines, histamine etc.)?
Ensure the bad stuff gets out.
In the context of these two questions, causes and contributing factors leading to clinical imbalances are identified and corrected.
We do not accept the conventional thinking that autism is an entity onto itself causing disease in the individual. Instead, autism is viewed only as a label for a group of individuals who share similar abnormalities in development and behaviors. By providing the body and brain with what they need and eliminating that which may be interfering, the potential exists to significantly improve brain function and improve the quality of life for these individuals.
This approach to medical practice allows us to offer gentle and supportive therapies to restore balance and allow the body’s natural healing ability to become our partner in achieving optimal health and wellness.
Autism Spectrum Disorder Causes and Contributing Factors
A large and rapidly growing body of research points towards ASD as an underlying genetic vulnerability which is then activated by environmental triggers. The combination of these two components then become the causes and/or contributing factors which we connect to the patient’s existing psychiatric diagnosis of ASD. There are a myriad of biomedical factors affecting the brain function of patients with autism. Most common contributing factors are listed below:
- Nutrition – deficiencies (particularly zinc, magnesium, B vitamins, vitamin D, vitamin A, antioxidants and omega-3 fatty acids) or increased needs from genetic mutations in biochemical pathways or poor intestinal absorption
- Food sensitivities/intolerances – particularly gluten and casein, which can produce opiate- like byproducts in susceptible patients
- Altered intestinal permeability
- Intestinal dysbiosis – pathological alterations in the gut microbiome
- Impaired methylation/transsulfuration
- Impaired detoxification
- Chronic inflammation
- Oxidative stress
- Mitochondrial dysfunction
- Autoimmunity or immune dysfunction
- Impaired production of hormones and/or neurotransmitters
One or more of these factors are present in almost all individuals with autism. All of these factors are functionally interrelated and most patients will have multiple factors contributing to their autistic behaviors. Some patients have all of them.
 Nutrition: begins with intake, however there is more to this than diet alone. Nutrition is what an individual eats, absorbs and delivers to their cells. Problems may start at the very beginning of this pathway with restricted appetite, food choices and overall poor intake or at the very end with certain mutations affecting adequate absorption of nutrients into cells of the brain. Many biomedical factors must be assessed such as reflux, constipation and zinc deficiency resulting in poor taste perception. Disruptions in sensation which can affect the taste, texture, smell and sight of food are also common in autism. Ensuring nutrients are at adequate levels for the individual, not just bare minimum norms determined for the population as a whole, can help support more optimal functioning. Many individuals with autism present with a variety of nutrients at deficient or suboptimal levels. Blood tests and functional urine testing can help identify these deficits or inefficiencies.
Nutrition: begins with intake, however there is more to this than diet alone. Nutrition is what an individual eats, absorbs and delivers to their cells. Problems may start at the very beginning of this pathway with restricted appetite, food choices and overall poor intake or at the very end with certain mutations affecting adequate absorption of nutrients into cells of the brain. Many biomedical factors must be assessed such as reflux, constipation and zinc deficiency resulting in poor taste perception. Disruptions in sensation which can affect the taste, texture, smell and sight of food are also common in autism. Ensuring nutrients are at adequate levels for the individual, not just bare minimum norms determined for the population as a whole, can help support more optimal functioning. Many individuals with autism present with a variety of nutrients at deficient or suboptimal levels. Blood tests and functional urine testing can help identify these deficits or inefficiencies.
– B Vitamins: unmet needs are common in autism. These vitamins play a critical role in human biochemistry by supporting energy metabolism, neurotransmitter synthesis, fat & protein metabolism, nerve function, brain health and overall health. Particularly B1, B2, B3, B6, B12 and folate.
– Vitamin A: antioxidant important in immune function and vision, including eye contact. Deficiency occurs in a subset of patients with autism.
– Vitamin D: deficiency is common in the general population as a whole. When present with autism, will exacerbate other clinical imbalances – particularly in relation to cognition and the immune system.
– Zinc: deficiencies are common in autism. This can be due to use of antacid medications, high glycemic diets, poor dietary intake of animal protein and the presence of toxic metals. Zinc is an essential cofactor in over 200 enzymatic reactions in the body which play roles in immune function, vitamin A transport, amino acid metabolism, toxic metal detoxification and taste perception.
– Magnesium: deficiency is common in the general population as whole and is seen even more in individuals with autism. Magnesium is an essential cofactor in over 300 enzymatic reactions in the body which play roles in energy (ATP) production, neurotransmitter function, methylation, transsulfuration and glutathione metabolism. Magnesium deficiency can result in many symptoms seen with autism, such as hyperactivity, anxiety, poor sleep and constipation.
– Omega-3 Fatty Acids: The Standard American Diet (SAD) provides excess pro-inflammatory omega-6 fatty acids and inadequate anti-inflammatory omega-3 fatty acids. Individuals with autism on a SAD can create a pro-inflammatory state in their bodies which could exacerbate other clinical imbalances. Omega-3 fatty acids, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have numerous health benefits.- Food sensitivities/intolerances: a significant number of individuals with autism are sensitive or intolerant of certain foods. While some may have traditional allergies (IgE mediated – what is tested at an allergist’s office), many more will have IgG mediated sensitivities which can result in physical, neurological and behavioral symptoms. IgG mediated food sensitivities differ from traditional food allergies in that they can occur in more delayed timeframe – up to 72 hours after ingestion of the sensitized food. IgG food sensitivity testing can give some indication of immunological reactions to food but the gold standard for diagnosis is an Elimination Diet. This consists of total elimination of the suspected offending food for several weeks followed by re-challenge with that food. Food sensitive individuals will have improvements with elimination of the food and worsening with reintroduction. Common food sensitivities seen in individuals with autism are casein (a dairy protein) and gluten (a protein found in wheat, barley and rye). Food sensitivity will typically affect brain function through altered intestinal permeability (also known as ‘leaky gut’). Offending foods can disrupt the membranes of the intestine allowing incompletely digested molecules to enter the bloodstream. Some of these undigested molecules are able to cross the blood-brain barrier and impact optimal brain function in many different ways including chronic inflammation, immune system dysregulation, opioid- like peptides and false neurotransmitters. A large number of individuals with autism exhibit food sensitivities. If the offending food or foods can be identified and eliminated, dramatic improvements usually follow.
- Intestinal dysbiosis: an imbalance in the intestinal micro biome. A rapidly growing body of research is documenting the close interrelationship of intestinal function and brain health. The intestine is home to trillions of beneficial bacteria that serve many useful functions including immune system regulation, production of nutrients, reduction of inflammation, improved digestion and absorption, and inhibition of the growth of harmful microbes. The main risks for developing dysbiosis are antibiotic use and inadequate dietary fiber. Through probiotics, changes in diet and other methods, the microbiome can be rebalanced leading to positive, visible changes in brain health and intestinal function.
- Impaired methylation/transsulfuration: the folate, methylation and sulfation cycles are interconnected biochemical pathways which serve vital functions in DNA synthesis, gene expression, neurotransmitter metabolism, antioxidant support and detoxification. These pathways are dependent on many vitamins and minerals, particularly folate, B12 and B6. These pathways often function poorly in individuals with autism. Measurement and correction of the imbalanced metabolites by appropriate supplementation is foundational to the successful biomedical treatment of autism.
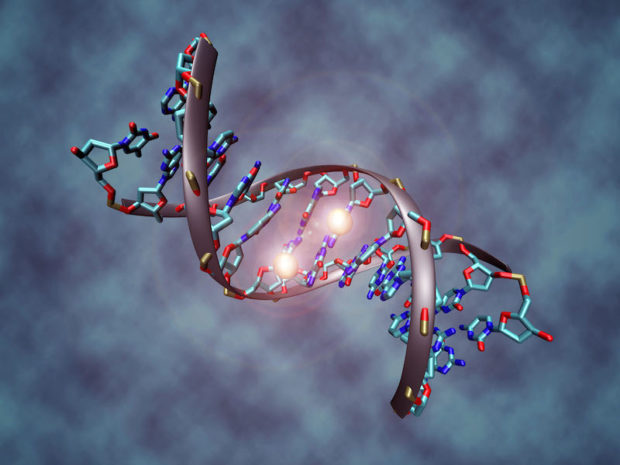
- Impaired detoxification: in some individuals detoxification can be decreased secondary to a physiological imbalance, genetic mutation or may be simply overwhelmed by the sheer number of toxins present in the modern environment. It may be caused by something as simple as constipation. In addition, many individuals with autism have impaired glutathione production. Glutathione is the body’s ultimate antioxidant and an essential factor for many detoxification processes within us. Deficiencies in the methylation/ transsulfuration cycle can adversely affect glutathione production and in the presence of these deficiencies, toxins may not be eliminated efficiently resulting in negative consequences on brain functioning, mitochondrial health and overall increases in oxidative stress and inflammation. These individuals will require assistance for optimal detoxification. Detoxification is a subject largely ignored in medical school, but there are many techniques available to remove toxins from the body, including oral and intravenous therapies.
- Mitochondrial dysfunction: mitochondria are within all cells of the body and are responsible for almost all of our energy production. Whereas a small subset of individuals with autism have outright mitochondrial disease, a much larger number have mitochondrial dysfunction. The difference between disease and dysfunction is in severity along a spectrum, much the same as autism. Mitochondrial dysfunction may be secondary to insufficient nutrients needed for optimal enzyme function or an inability of the individual to synthesize certain factors. Given their vital role in energy production, contributing factor can have far-reaching effects on overall health. Mitochondrial dysfunction can be managed and even reversed with nutritional support (supplements and targeted food plans), lifestyle modification (specific types of exercise, meal-timing & adequate sleep) and individualized neuro-rehabilitative techniques.
- Oxidative stress and inflammation: oxidative stress is a process which the body responds to stressors (i.e infection, toxins, trauma etc.) via the production of free radicals. The free radicals attack the stressor only when the system is in balance. When imbalanced, those same free radicals can result in damage to healthy cells and DNA. Imbalances may occur from a variety of reasons, the most common being chronic inflammation and insufficient levels of antioxidants. The negative effects of oxidative stress have been observed on the functioning of brain glial cells. Glial cells provide nourishment to neurons and clean up toxic byproducts. When glial cell function is poor, negative effects are noted on brain function. Poor language, poor sensory processing and overall suboptimal brain function has been noted when these imbalances persist. Most of the other contributing factors directly or indirectly result in oxidative stress and correction of this imbalance is crucial. Finding the root cause and reversing excess oxidative stress is one of the first steps in the biomedical management of autism.

- Autoimmunity or immune dysfunction: a variety of immune system dysfunctions has been documented in autistic individuals. Increases in pro-inflammatory cytokines, decreases in natural killer cell activation, abnormal levels of immunoglobulin and brain autoantibodies have all been found in the brains of individuals with autism. This is one of the newest areas of research in treatment of autism and our knowledge of the imbalances in these areas is rapidly growing.
- Imbalanced hormones/neurotransmitters: there is a huge diversity of these imbalances between different individuals with autism. Many can be corrected by balancing other issues (such as food sensitivities, methylation, immune dysfunction etc.) whereas others may require direct supplementation (such as oxytocin which is also known as “the bonding hormone” or “love hormone”).

If you are looking for an autism doctor in Las Vegas, then please contact us here. Dr. Armen Nikogosian treats autistic patients throughout the Las Vegas valley. His office is located in Las Vegas, NV. He is also available for remote consultations.
References
The Autism Biomed Center approach is science-based and all the information presented here has been drawn from various peer-reviewed scientific literature cited below.